So you want some ankle replacement pictures or to choose the best implant for you as well as the pros and cons of ankle replacements. Its really hard to find useful and reliable information on the Internet about the different types of total ankle replacement. In this article we discuss some of the different ankle replacement pictures, implants and prostheses on the market to help you with your ankle replacement recovery.
THE INFINITY ANKLE REPLACEMENT
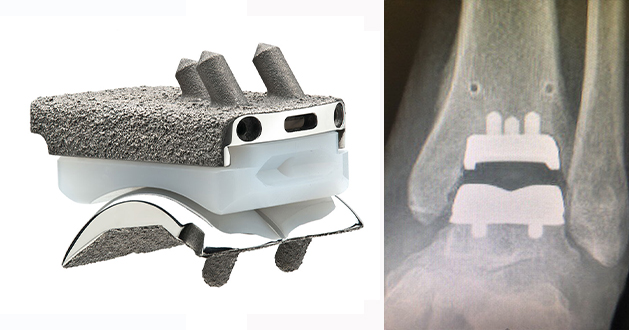
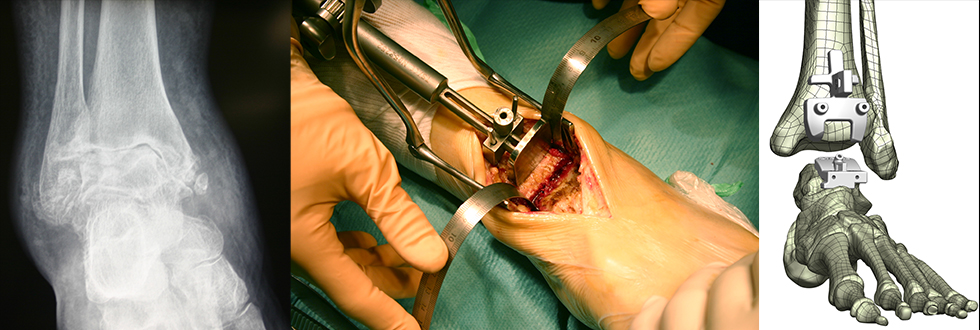
The Infinity™ ankle replacement is the commonest used implant globally and the commonest ankle replacement in the UK. The implant was launched by Wright Medical in 2014 but in 2020 Wright Medical was acquired by Stryker Inc and so they now own the implant. The Infinity ankle replacement is a two component fixed bearing prosthesis made of titanium or cobalt chrome with a porous titanium plasma spray coating. There is quite a large number of publications to date but the largest outcome series is a multicentre, non-inventor, prospective observational study of 504 implants. All patients had improvement in clinical scores at 6 months, maintained up to 2 year follow up but only 104 patients had reached this milestone at initial reports in 2020 so its important to watch this space.
The Infinity™ implant can be used with standard instruments or using a technique known as Patient Specific Instrumentation (PSI) also known as Prophecy™ which is customised to the patients anatomy based on a CT scan performed prior to the surgery.
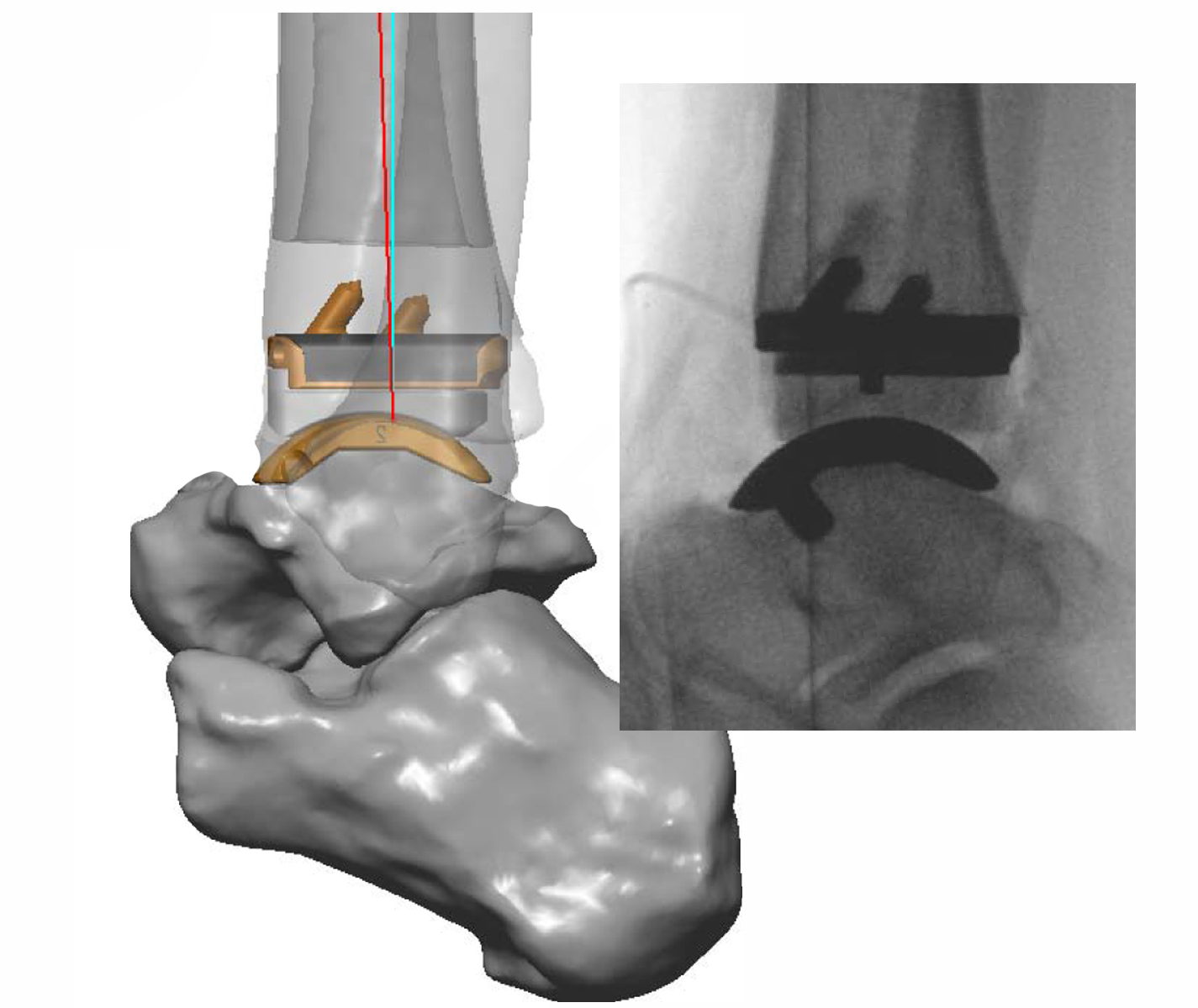
The Prophecy Infinity Ankle Replacement
THE CADENCE ANKLE REPLACEMENT
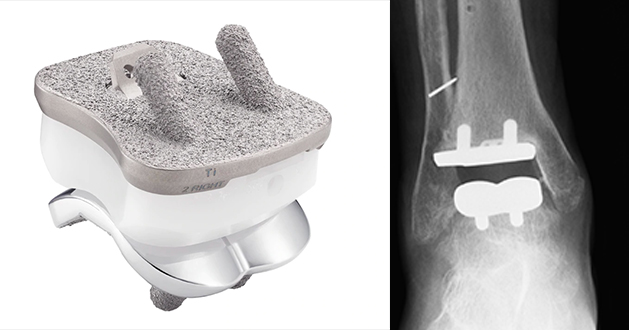
The Cadence™ ankle replacement is distributed by Integra LifeSciences. It was launched in 2016 and is a two part fixed bearing prosthesis made of titanium alloy and cobalt chrome alloy. There is limited published outcome data outside of the 2 year surgeon designer series.
THE HINTEGRA (H2/H3) ANKLE REPLACEMENT
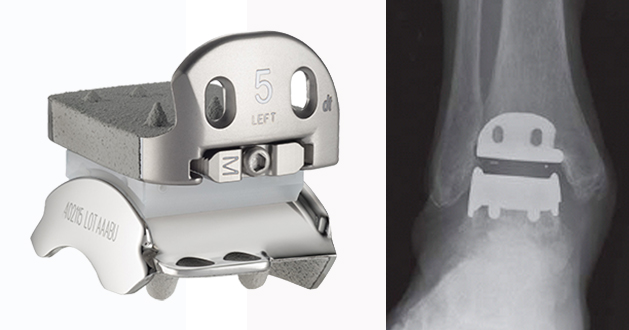
The Hintermann Series Total Ankle Replacements began life as the Hintegra distributed by Integra Lifesciences with the first implant in 2000. In 2016 ownership moved to DTMedtech LLC who received FDA premarket approval for a two component version, entitled the H2™. DT Medtech LLC then used 18 years of non US data of their three component design to obtain FDA premarket approval of the H3 prosthesis which was granted in June 2019. The implant has a lot of published data the largest series of which analysed 722 implants at a mean follow up of 6 years. The data needs to be carefully interpreted since the Indications for Use (IFU) differs for the H2™ and H3™ depending on whether it is sold in the USA or outside of the USA.
THE STAR ANKLE REPLACEMENT
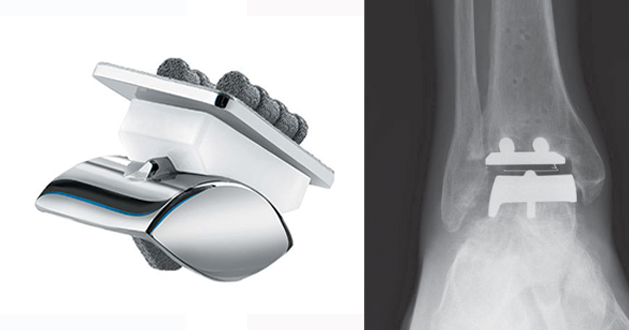
The STAR™ ankle stands for Scandinavian Total Ankle Replacement. It was developed by Hakon Kofoed in Denmark and first implanted in 1981. Since that time there have been more than 5 different versions of the STAR prosthesis. The first four were used in Europe whereas the fifth variety was introduced into the US market in 1998 and FDA approved in 2009.
Although the first STAR™ ankle was a 2 component cemented design and was implanted between 1981–1986, Kofoed then developed the 3 component mobile bearing implant to replace this from 1986–2012. In 1989 the 3rd generation 3 piece design but with HA coating was introduced. Kofoed published improved results from the 3rd generation implant with 12 year survival of 95.4%.
In 1998, the base coating of the STAR implant was changed to a rough Titanium plasma so that it could be used in the USA. This is the design used on the US clinical trials for FDA approval (Mann et al., 2011) and is the current design used in the USA & Canada. This is different than the current design used outside of the USA and Canada, as of 1999, which has Calcium Phosphate on top of the Titanium plasma spray (referred to as double coat).
THE VANTAGE ANKLE REPLACEMENT
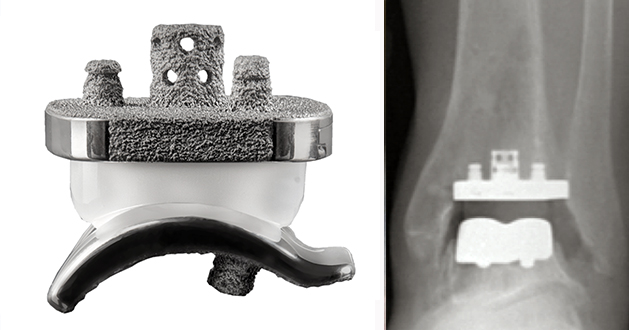
The Vantage™ ankle replacement is manufactured by Exactech Inc. It was launched in 2017 and is a two part fixed bearing prosthesis in the USA but a three component mobile bearing prosthesis outside of the USA. It is made of Stainless steel Nitronic 60 and the tibial component has a vertical cage pressfit for initial fixation and peripheral pegs for rotational stability. There is limited published outcome data yet.
Did you enjoy this article? You may also like; best ankle replacement surgeons
Ankle replacement vs ankle fusion
Jane’s story – the arthritis fix that finally took away my agony